Smart Solutions to Make Products Consumers Can Trust
Check, improve and certify your product quality and supplier compliance.
Book online and gain real-time insights.
Get Started► See us in action
Improve Your Supply Chain Globally
Control your product and supplier quality with QIMA qualified inspectors, auditors and labs
Improve quality and compliance with technical support from our industry experts
Trusted by 15,000+ brands, retailers and importers
Intelligent Platform for Quality
Collect quality data from the field
Manage and roll out consistent quality protocols with your suppliers
Get custom analytics, benchmark and improve your suppliers' performance
Use QIMAone: Our SaaS platform for quality management
The QIMA Difference
Top-rated customer service1: 24/7/365 chat support and dedicated contacts
Flexibility: build custom quality control and assurance programs
Reactivity: onsite in 48 hours, fastest report turnaround time
(1) Client survey (n=200).QIMA customer service rated +13% above competition.
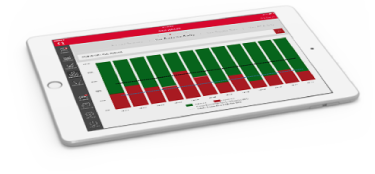


The Online Advantage
Manage all your services with QIMA from the convenience of your online account and the QIMA App
- Book orders in minutes
- Review, share, approve & reject reports with your suppliers
- Get your dashboard of quality and supplier KPI's, benchmark within your industry
- Manage your quality documentation online
- Get instant support from QIMA, 24/7/365 via our chat interface

QIMAone: our SaaS Platform for Quality and Compliance Management
- Equip your own inspectors and suppliers with our cloud-based inspection software
- QIMAone gives you visibility, agility and control to improve your quality performance
Services for Your Industry
QIMA has quality and compliance experts for all consumer goods and food categories. With over 2,500 inspectors, auditors and lab experts, we can help you assess and improve your product and supplier quality and safety performance. Access hundreds of off-the-shelf customizable inspection checklists online, receive expert advice on your product safety requirements and improve supplier compliance at the source.
QIMA Webinars
Check out our coming webinars and sign up to hear from our experts on supply chain, quality and compliance topics.

April 30th, 2024
Understanding PFAS: A Glimpse into the Latest Regulatory Amendments in the EU and U.S. (EU/U.S. Session)

April 30th, 2024
Understanding PFAS: A Glimpse into the Latest Regulatory Amendments in the EU and U.S. (Asia/EU Session)

On Demand
Navigating SVHC Presence in Consumer Products – Regulatory Requirements, Common Failure and Test Methods